Hep B biotech Antios closed after FDA hold proved insurmountable
Por um escritor misterioso
Descrição
Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable. | Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable.

IHEP (International Hepatology Education Program)

Core Concepts - Hepatitis B Coinfection - Co-Occurring Conditions

Microfluidic Formulation of Topological Hydrogels for Microtissue
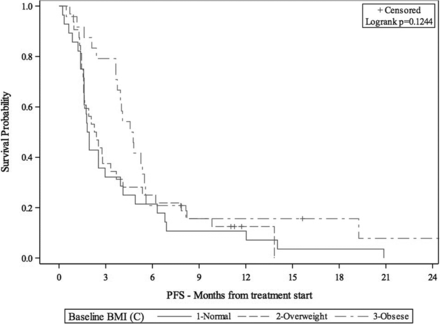
33rd Annual Meeting & Pre-Conference Programs of the Society for

Can Medivation's CEO get a bidding war started?
33rd Annual Meeting & Pre-Conference Programs of the Society for
FDA Places Clinical Hold on Antios' HBV Therapy, Ending

Strategies to Control or Mimic Growth Factor Activity for Bone
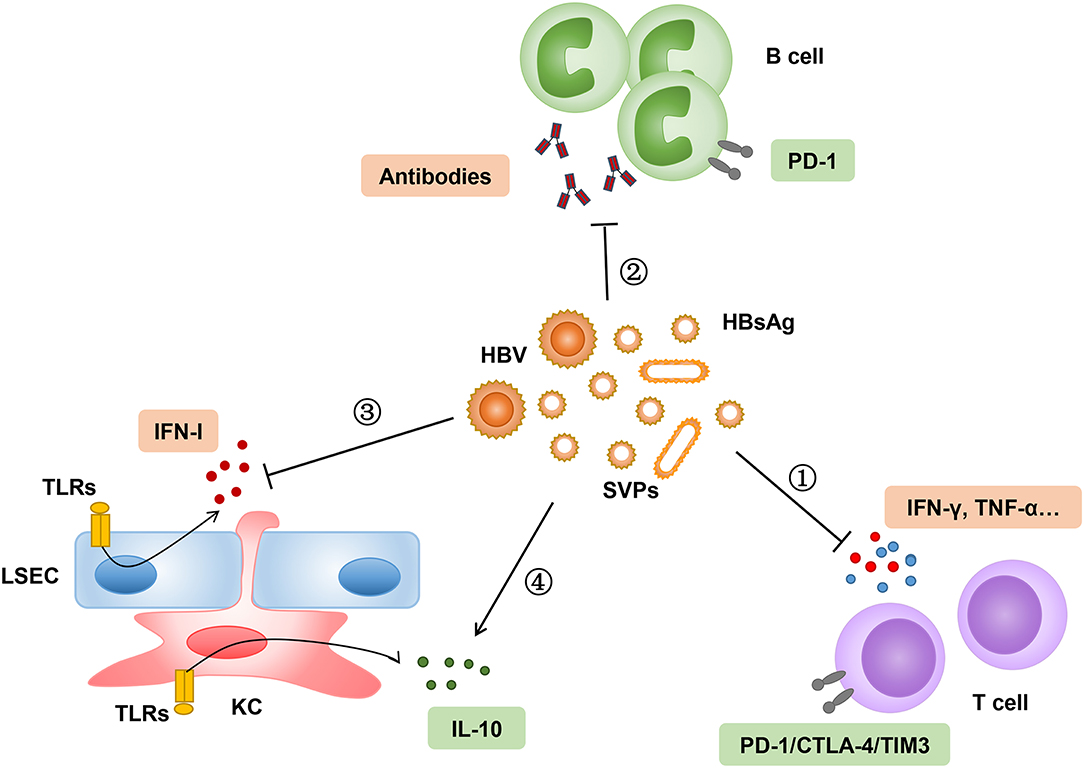
Frontiers Toward a Functional Cure for Hepatitis B: The

HBV replication inhibitors. - Abstract - Europe PMC

Biotech Fierce Biotech
Assembly Bio breaks off hepatitis B deal with Antios Therapeutics
Biotech Fierce Biotech
de
por adulto (o preço varia de acordo com o tamanho do grupo)