In Vitro vs In Vivo Preclinical Studies
Por um escritor misterioso
Descrição
Before a drug candidate can be tested in humans, its safety and efficacy must be explored in in vitro or in vivo preclinical studies.
The Pathophysiology of Neuropsychiatric Disorders Program
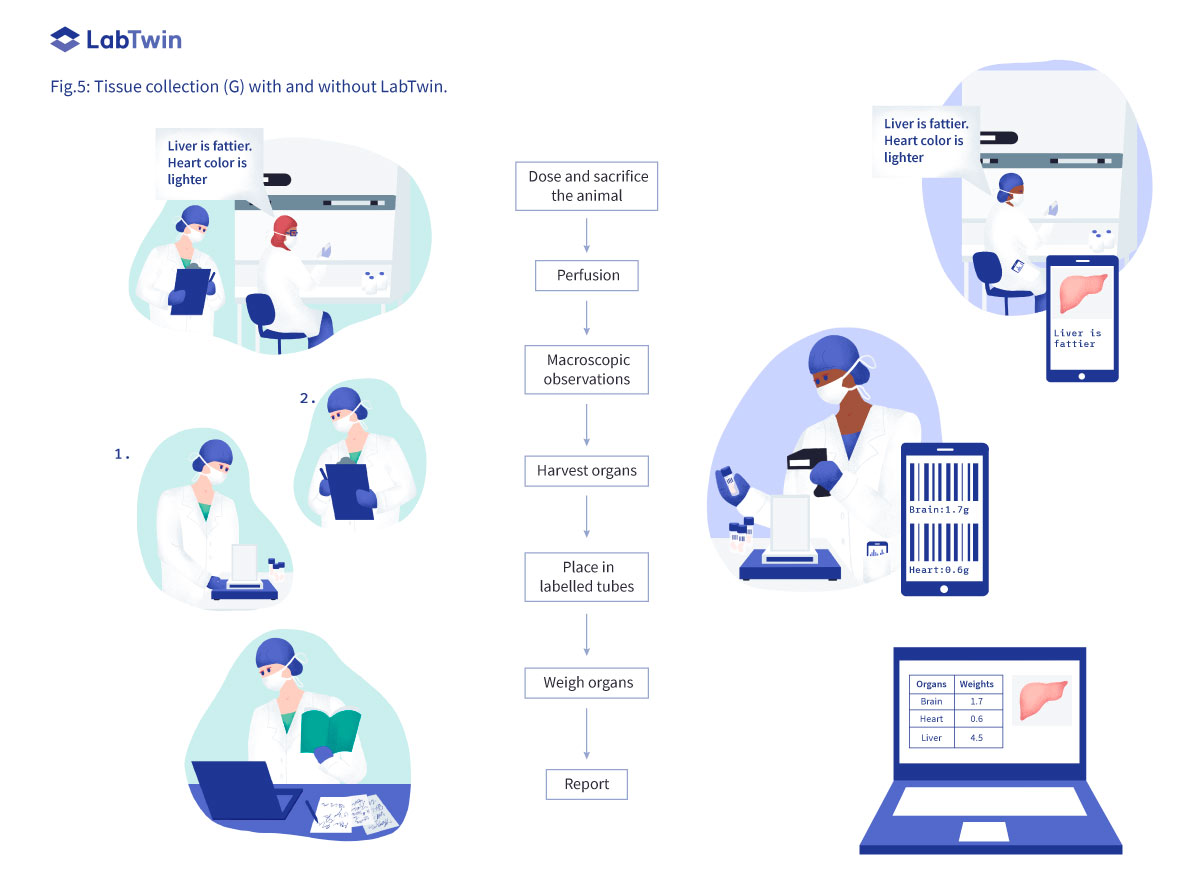
Documenting in vivo Preclinical Studies 2022

Extrapolation of in vitro data to preclinical and.pptx

in vivo preclinical studies for drug discovery

Exploring the Drug Development Process

Animal tests no longer necessary before drug clinical trials
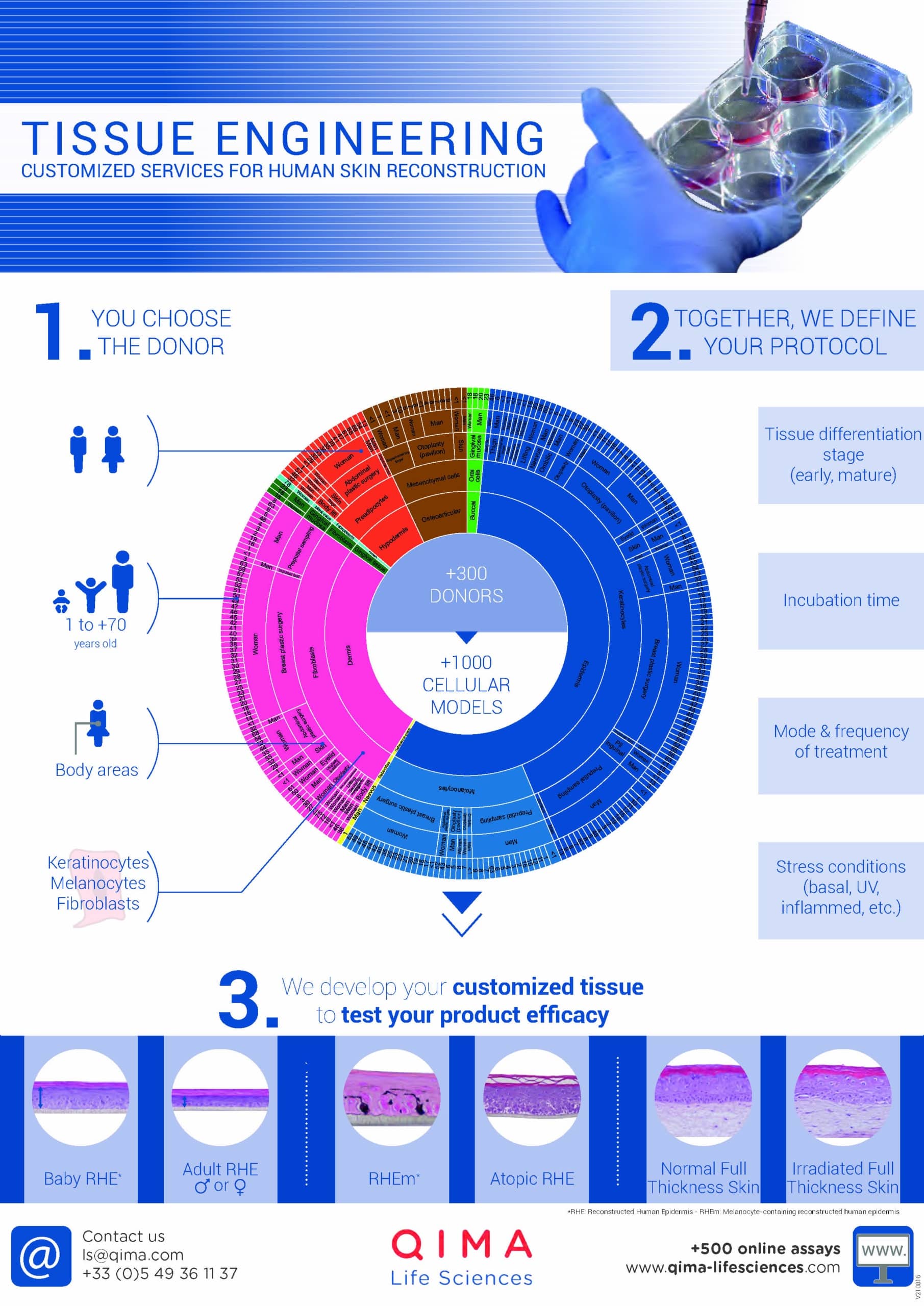
Differences Between Ex Vivo & In Vitro Models

Preclinical Studies for Safer Clinical Trials: Toxicokinetics

Understanding the correlation between in vitro and in vivo
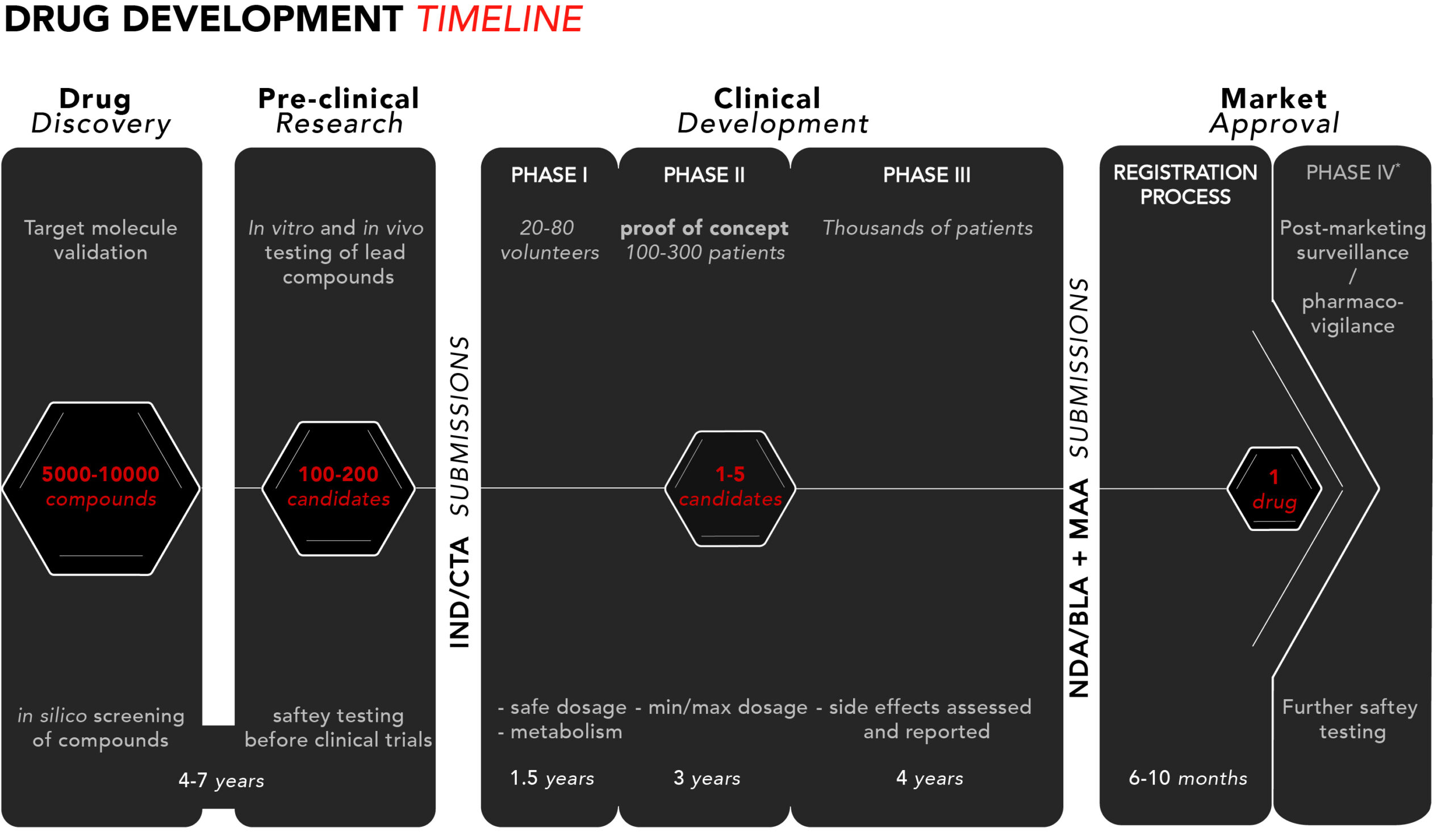
Drug development – The four phases - BioStock
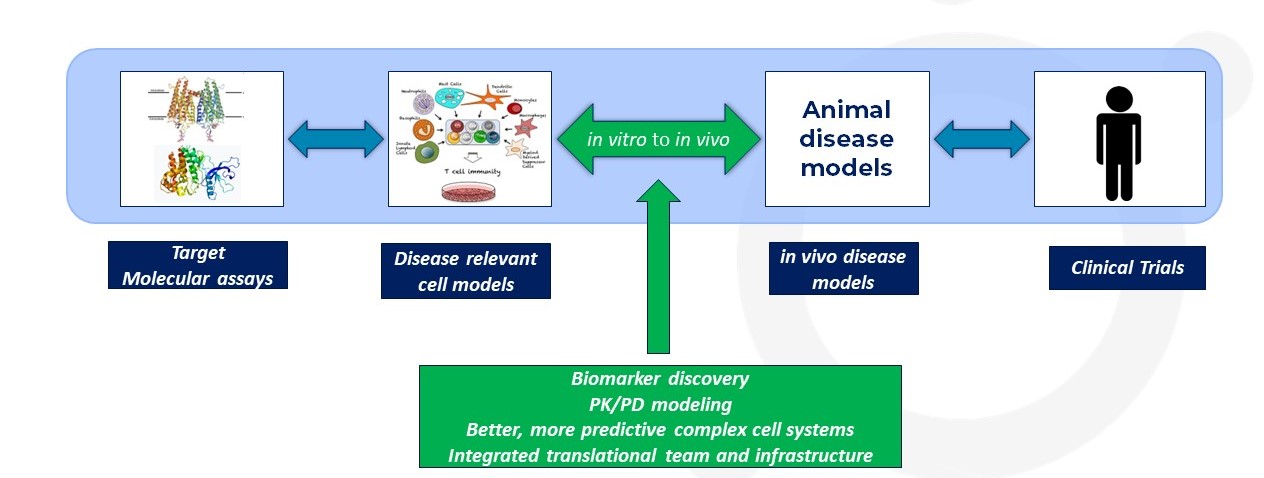
The Significance of In Vitro to In Vivo Translation in Drug Discovery

Baseline and time‐updated factors in preclinical development of
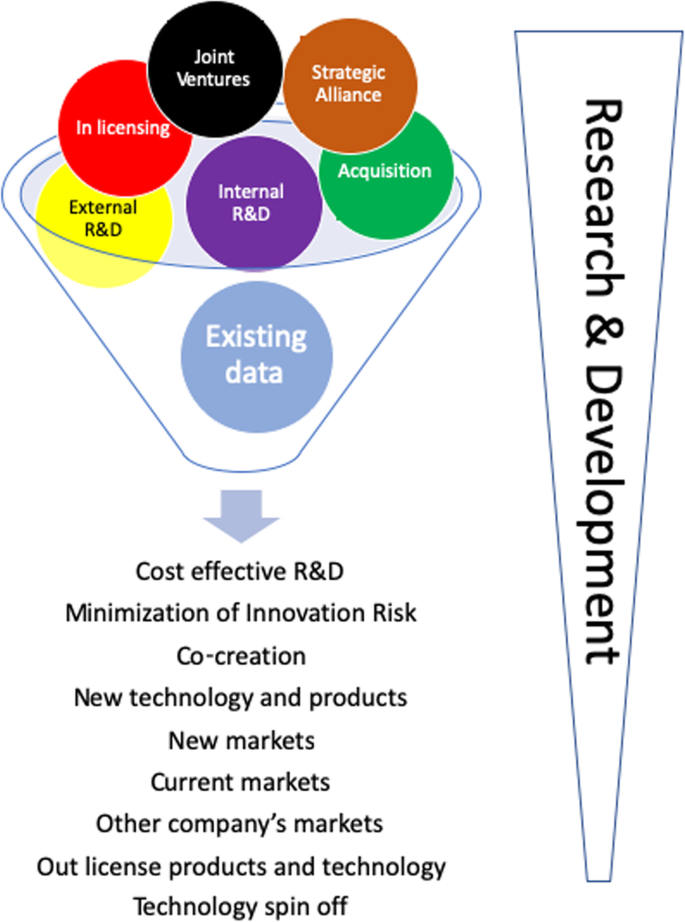
Lost in translation: the valley of death across preclinical and
de
por adulto (o preço varia de acordo com o tamanho do grupo)