Arsenic Trioxide Induces Apoptosis of Human Monocytes during Macrophagic Differentiation through Nuclear Factor-κB-Related Survival Pathway Down-Regulation
Por um escritor misterioso
Descrição
Arsenic trioxide (As2O3) is known to be toxic toward leukemia cells. In this study, we determined its effects on survival of human monocytic cells during macrophagic differentiation, an important biological process involved in the immune response. As2O3 used at clinically relevant pharmacological concentrations induced marked apoptosis of human blood monocytes during differentiation with either granulocyte-macrophage colony-stimulating factor or macrophage colony-stimulating factor. Apoptosis of monocytes was associated with increased caspase activities and decreased DNA binding of p65 nuclear factor-κB (NF-κB); like As2O3, the selective NF-κB inhibitor ( E )-3-[(4-methylphenyl)-sulfonyl]-2-propenenitrile (Bay 11-7082) strongly reduced survival of differentiating monocytes. The role of NF-κB in arsenic toxicity was also studied in promonocytic U937 cells during phorbol 12-myristate 13-acetate-induced macrophagic differentiation. In these cells, As2O3 first reduced DNA binding of p65 NF-κB and subsequently induced apoptosis. In addition, overexpression of the p65 NF-κB subunit, following stable infection with a p65 retroviral expressing vector, increased survival of As2O3-treated U937 cells. As2O3 specifically decreased protein levels of X-linked inhibitor of apoptosis protein and FLICE-inhibitory protein, two NF-κB-regulated genes in both U937 cells and blood monocytes during their differentiations. Finally, As2O3 was found to inhibit macrophagic differentiation of monocytic cells when used at cytotoxic concentrations; however, overexpression of the p65 NF-κB subunit in U937 cells reduced its effects toward differentiation. In contrast to monocytes, well differentiated macrophages were resistant to low concentrations of As2O3. Altogether, our study demonstrates that clinically relevant concentrations of As2O3 induced marked apoptosis of monocytic cells during in vitro macrophagic differentiation likely through inhibition of NF-κB-related survival pathways.

Macrophages in oxidative stress and models to evaluate the antioxidant function of dietary natural compounds - ScienceDirect

Graphene Oxide Induces Toll-like Receptor 4 (TLR4)-Dependent Necrosis in Macrophages
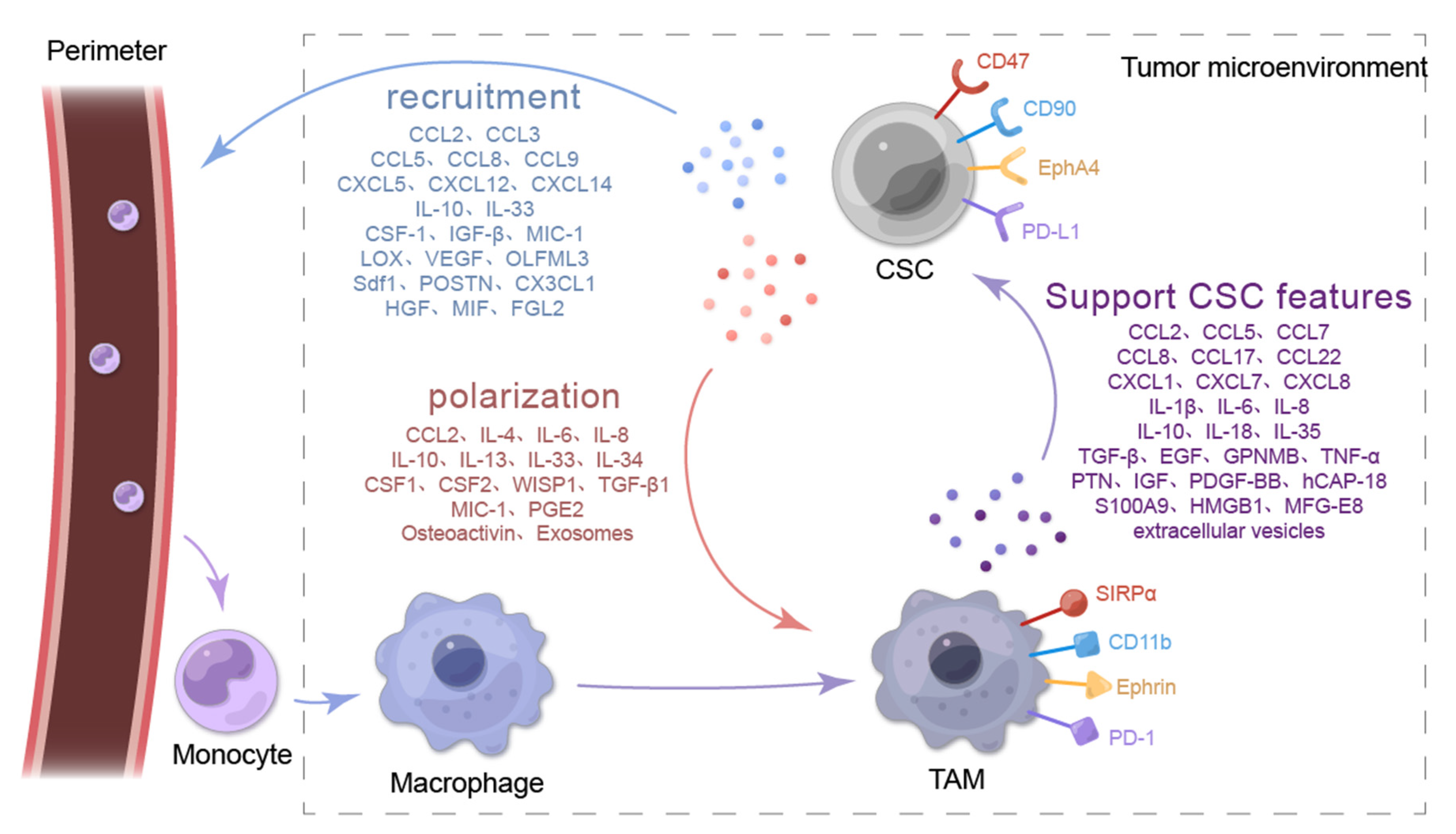
Biomolecules, Free Full-Text
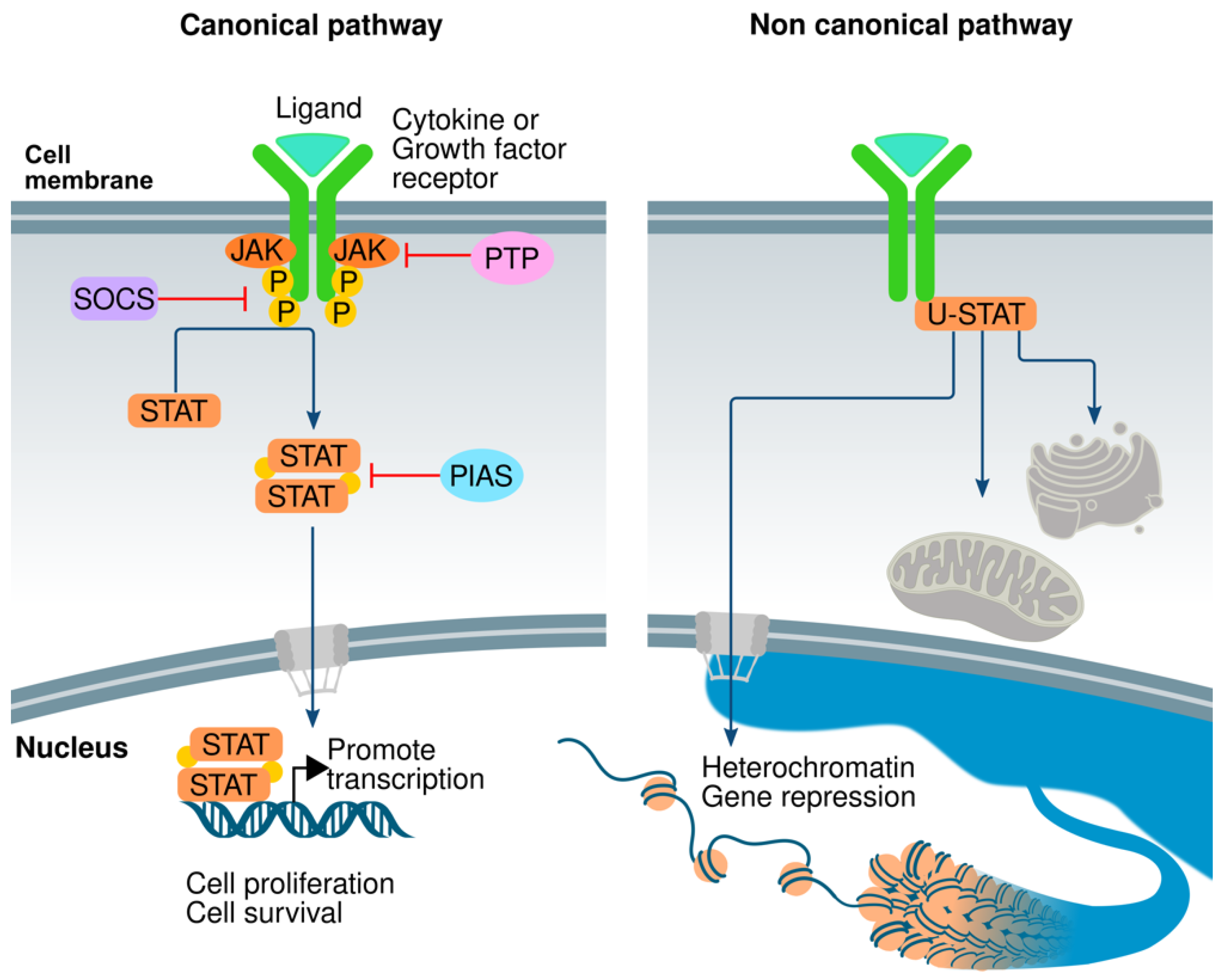
Genes, Free Full-Text

Arsenic Trioxide Induces Apoptosis of Human Monocytes during Macrophagic Differentiation through Nuclear Factor-κB-Related Survival Pathway Down- Regulation
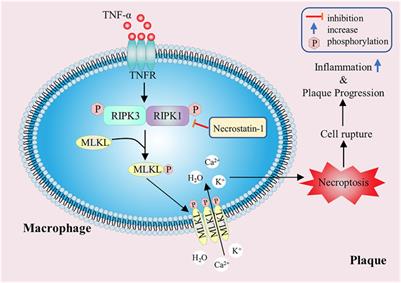
Frontiers The Function, Regulation and Mechanism of Programmed Cell Death of Macrophages in Atherosclerosis

Diagram depicts the toxic effect of arsenic trioxide on chicken immune
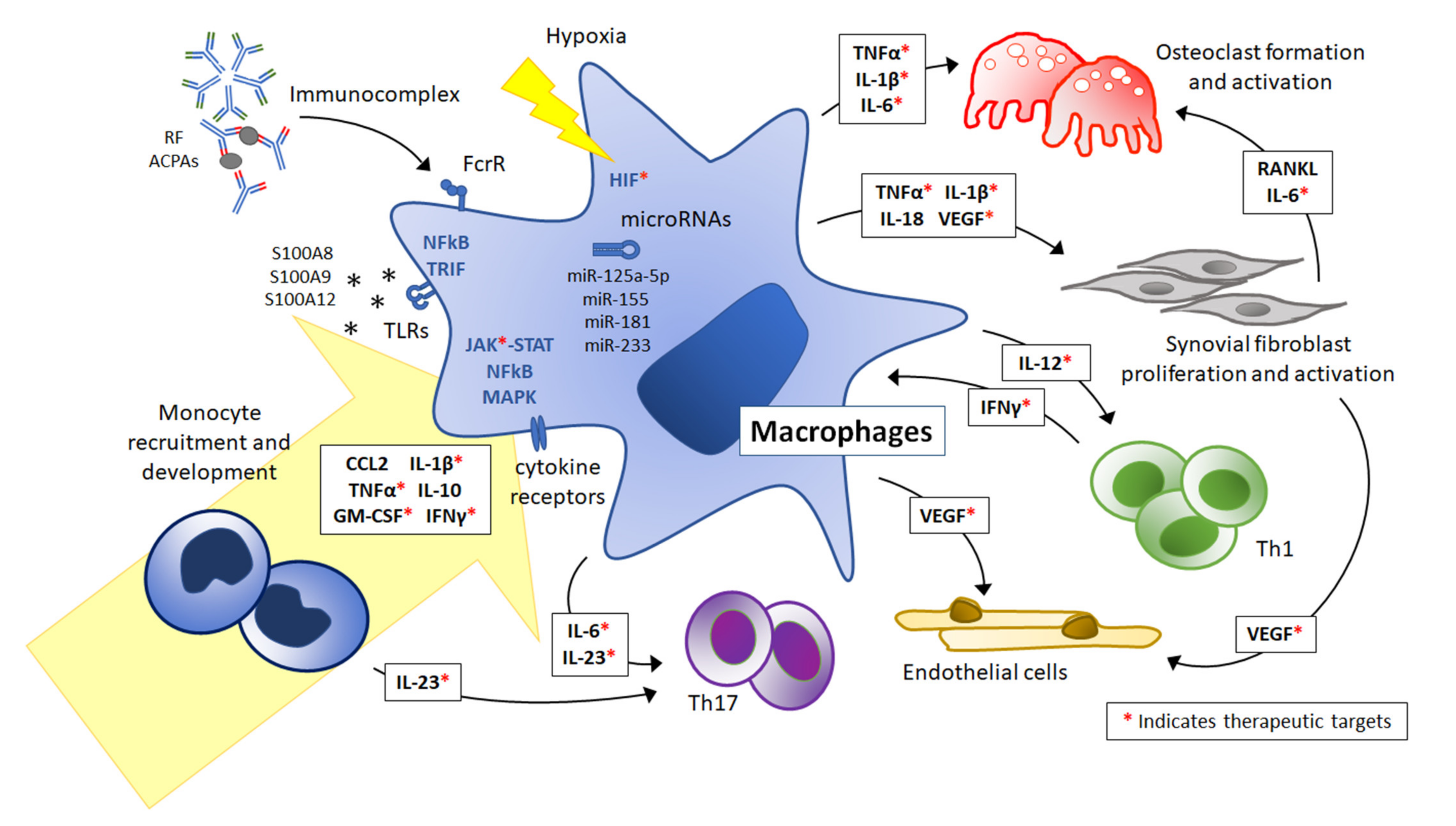
IJMS, Free Full-Text
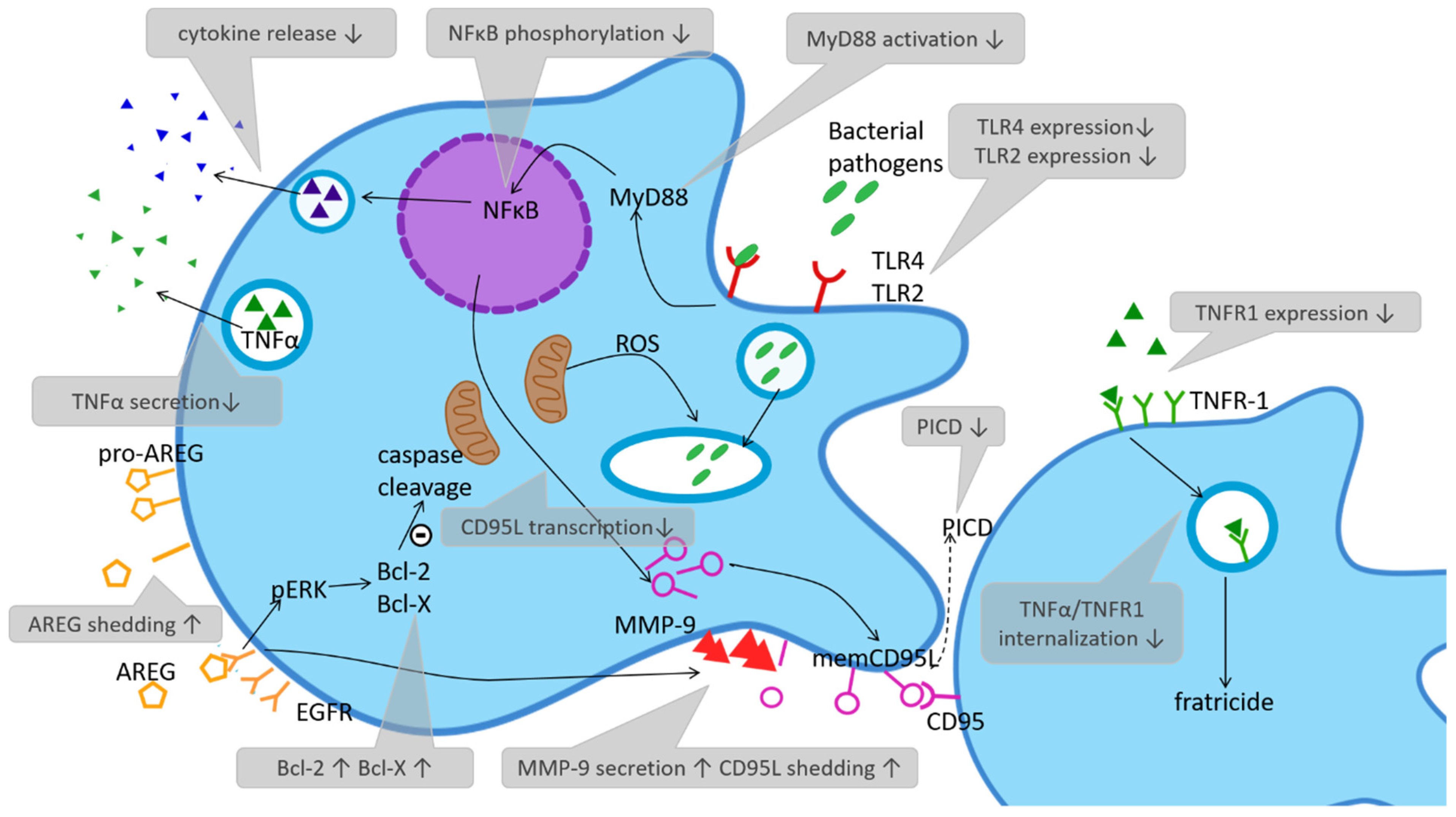
BioChem, Free Full-Text

Arsenic as an immunotoxicant - ScienceDirect

Regulated Necrosis in Atherosclerosis Arteriosclerosis, Thrombosis, and Vascular Biology
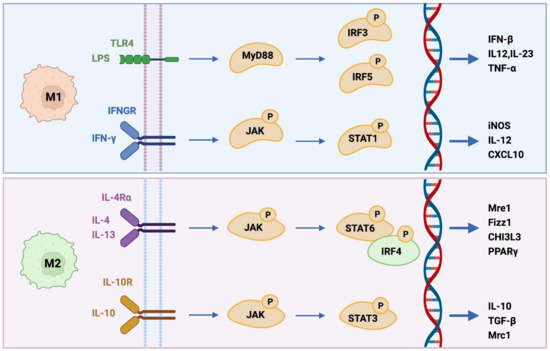
Cells, Free Full-Text
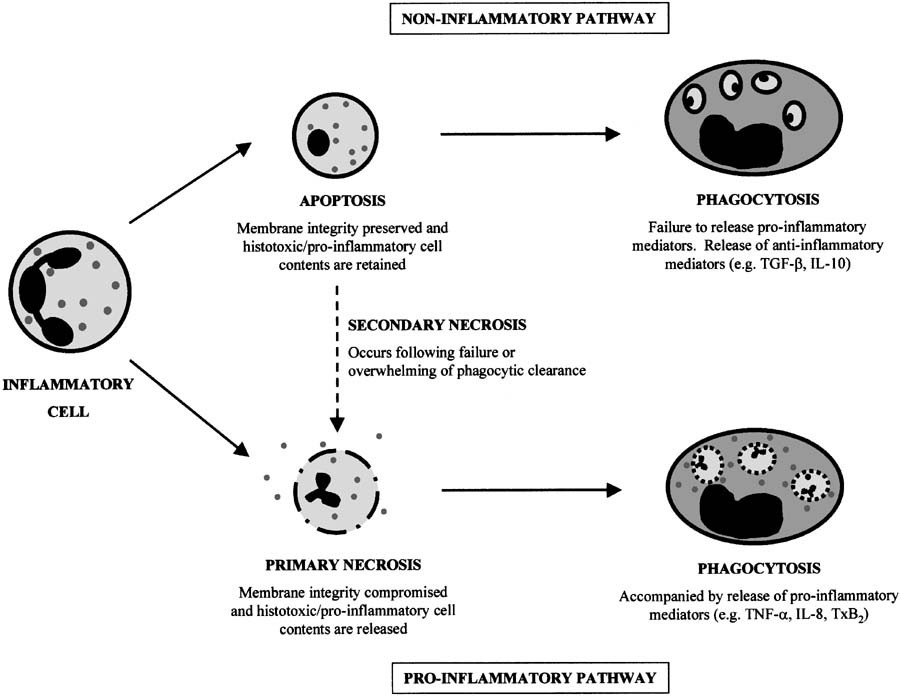
Nitric oxide: a key regulator of myeloid inflammatory cell apoptosis

Macrophage Reprogramming via Targeted ROS Scavenging and COX-2 Downregulation for Alleviating Inflammation

Lgr4 Governs a Pro-Inflammatory Program in Macrophages to Antagonize Post-Infarction Cardiac Repair
de
por adulto (o preço varia de acordo com o tamanho do grupo)