FDA OKs Cell Therapy to Lower Infection Risk After Stem Cell Transplant
Por um escritor misterioso
Descrição
Omidubicel reduced infections in blood cancer patients from 60% to 39% at 100 days posttransplant

Despite Progress, Government Can Better Protect Patients From
What are the differences between platelet rich plasma and stem
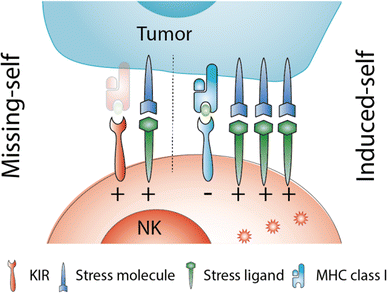
NK cell therapy after hematopoietic stem cell transplantation: can
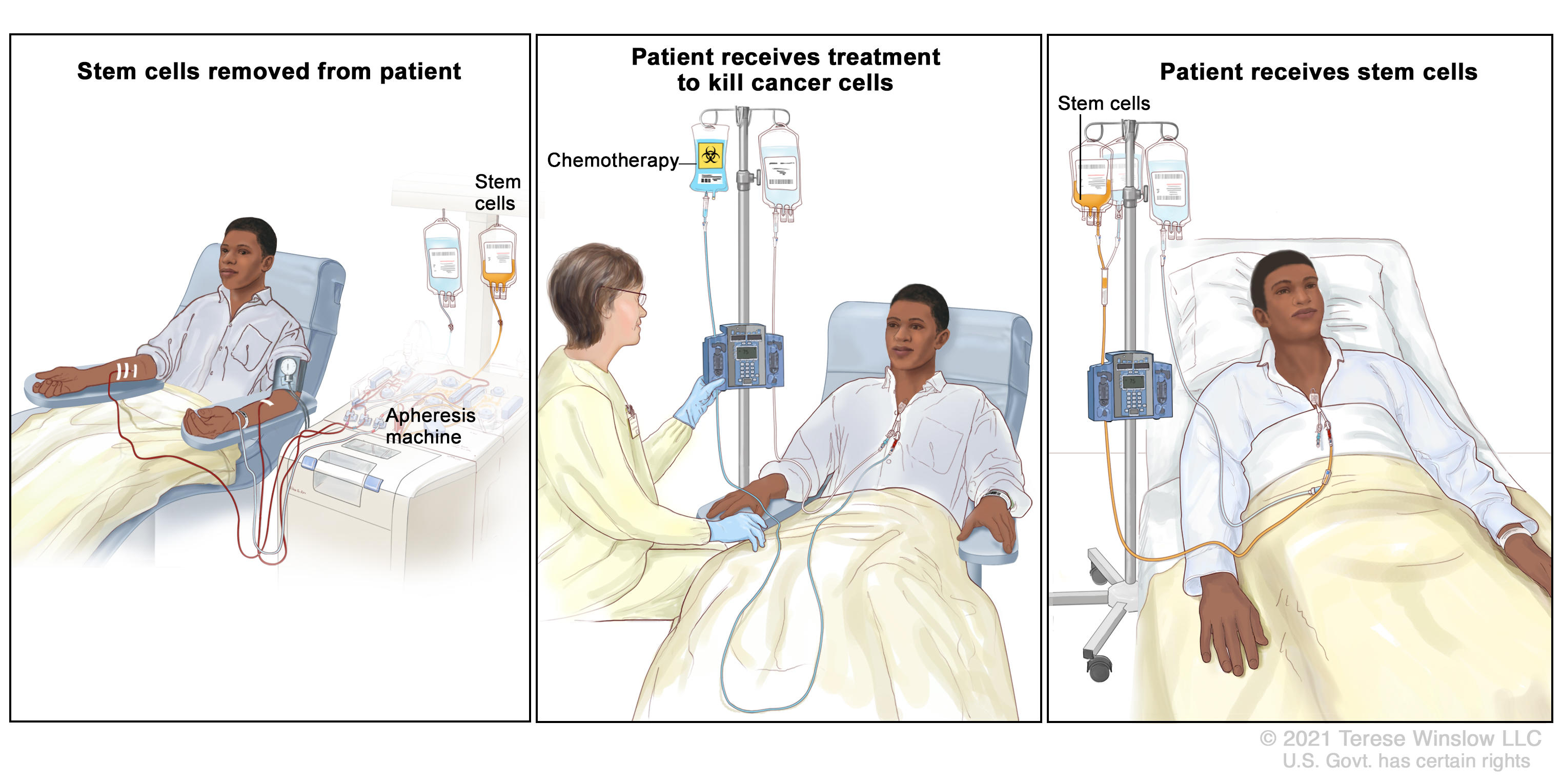
Stem Cell and Bone Marrow Transplants for Cancer - NCI
FDA Approves Cell Therapy for Blood Cancer Patients to Cut
Although Promising, CAR T-Cell Therapy in T-ALL Carries Many
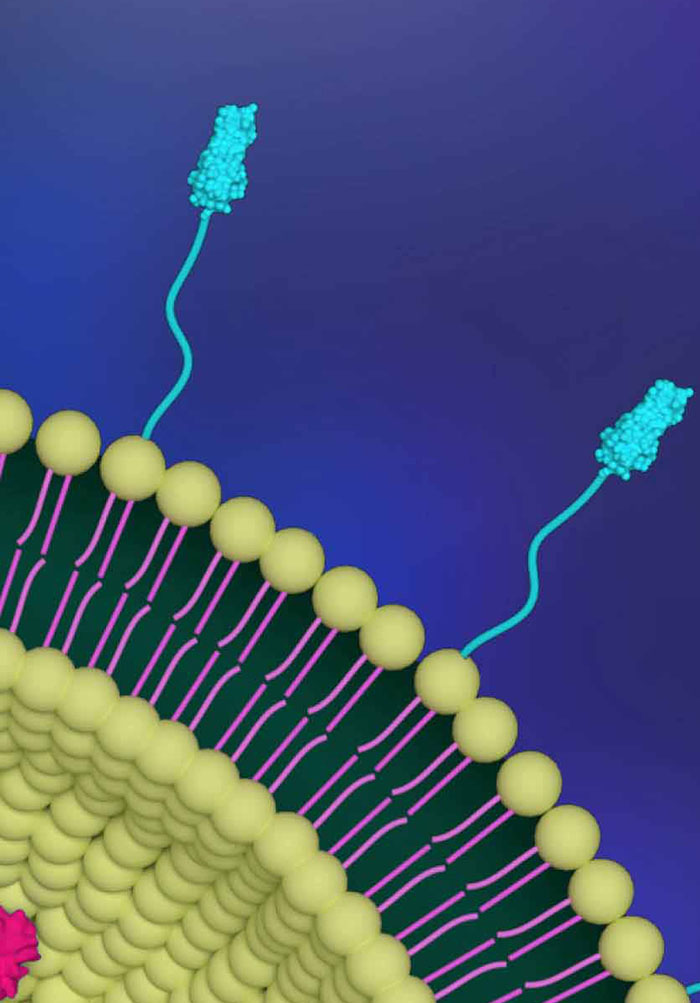
Perspective Chapter: Liposome Mediated Delivery of

Mesenchymal Stromal Cells: an Antimicrobial and Host-Directed
PDF) Nonmyeloablative Allogeneic Hematopoietic Stem Cell

Tool to easily search for a stem cell clinical trial for your

FDA Approves Cell Therapy for Patients with Blood Cancers to

FDA approves cell therapy for patients with blood cancers to
:max_bytes(150000):strip_icc()/Health-Stocksy-3418126-80bec79dd1a145419ff8a990f1b32b57.jpg)
FDA Approves Fecal Transplant Therapy for Recurrent C. Diff

FDA Approves Therapy to Decrease Infection Risk Following Stem
de
por adulto (o preço varia de acordo com o tamanho do grupo)







