What You Should Know About CSV in Pharma
Por um escritor misterioso
Descrição
Learn more about computer system validation, which is required by the FDA and other global regulatory bodies for drug and medical device manufacturers.

What is Computer System Validation and How Do You Do It?

Software Validation for IT Infrastructure - MasterControl
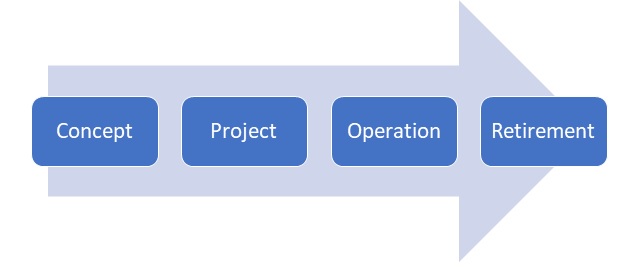
CSV Life Cycle - Gsap
Preparing for the GAMP Transition to Computer Software Assur

What You Should Know About CSV in Pharma

5 Steps to More Efficient Computer System Validation

COMPUTER SYSTEM VALIDATION [CSV] - pharma arena

9 of the Best Online CSV Resources for Pharma Validation Professionals

Software Validation / Computer System Validation (CSV) /

Computer System Validation (CSV) in Life Sciences Part 1: Introduction to CSV - Verista
de
por adulto (o preço varia de acordo com o tamanho do grupo)