What will be the shape of ICl 2 among the following?A. BentB. Trigonal planarC. LinearD. Trigonal bipyramidal
Por um escritor misterioso
Descrição
What will be the shape of ICl 2 among the following?A. BentB. Trigonal planarC. LinearD. Trigonal bipyramidal
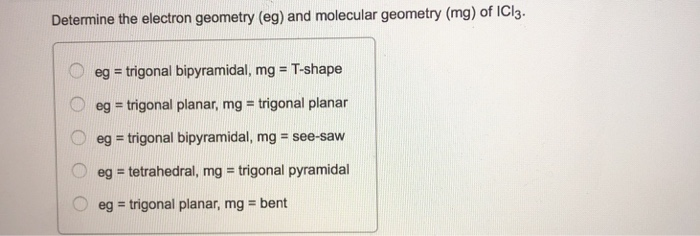
Solved Determine the electron geometry (eg) and molecular

Trigonal Bipyramidal Molecular Geometry/Shape and Bond Angles

Answered: Determine the electron geometry (eg)…
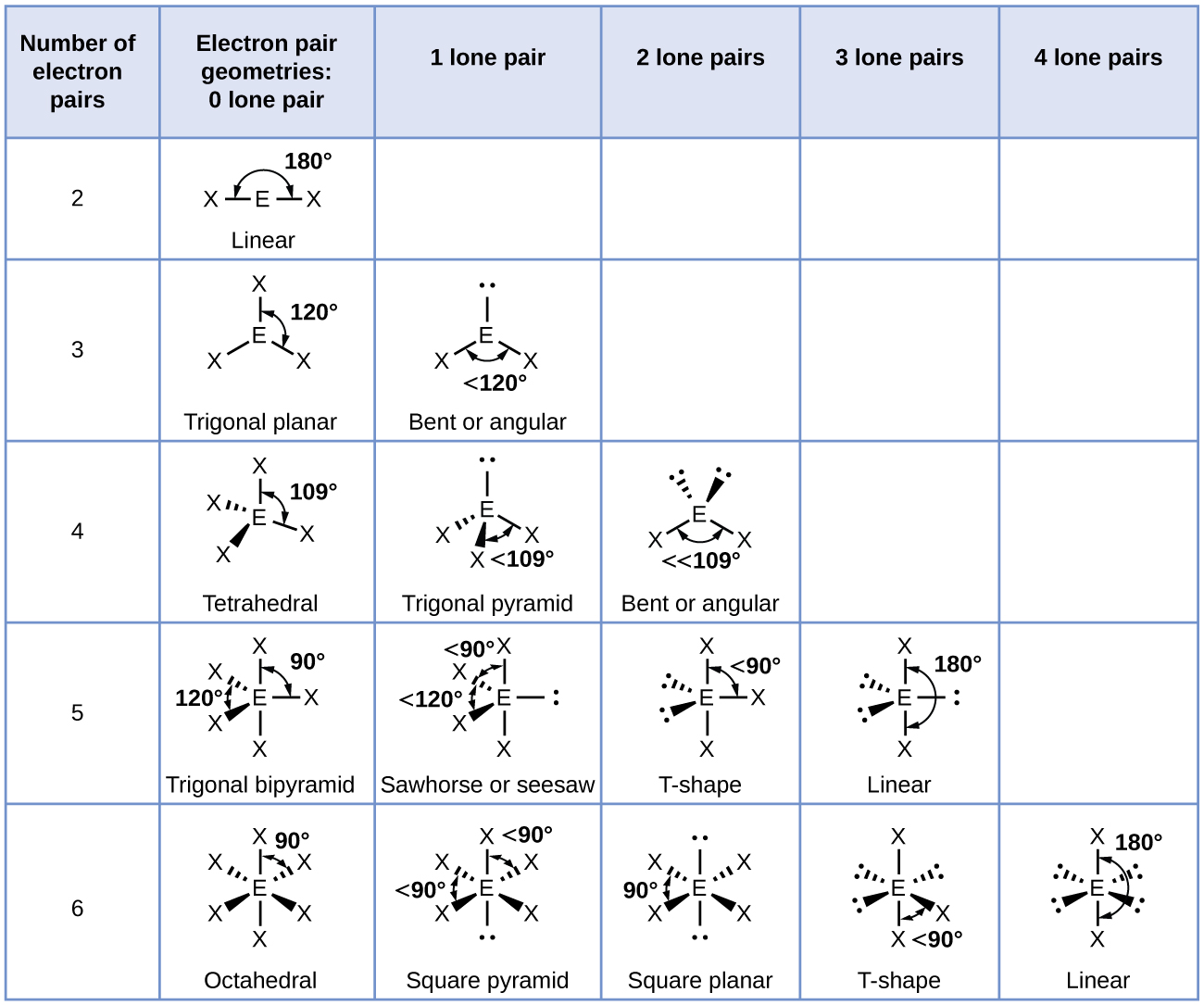
3.3 Molecular Structure and Polarity – Chemical Bonding and

Use the VSEPR theory to predict the shape of carbon tetrachloride

trigonal bipyramidal, seesaw, T shaped and linear
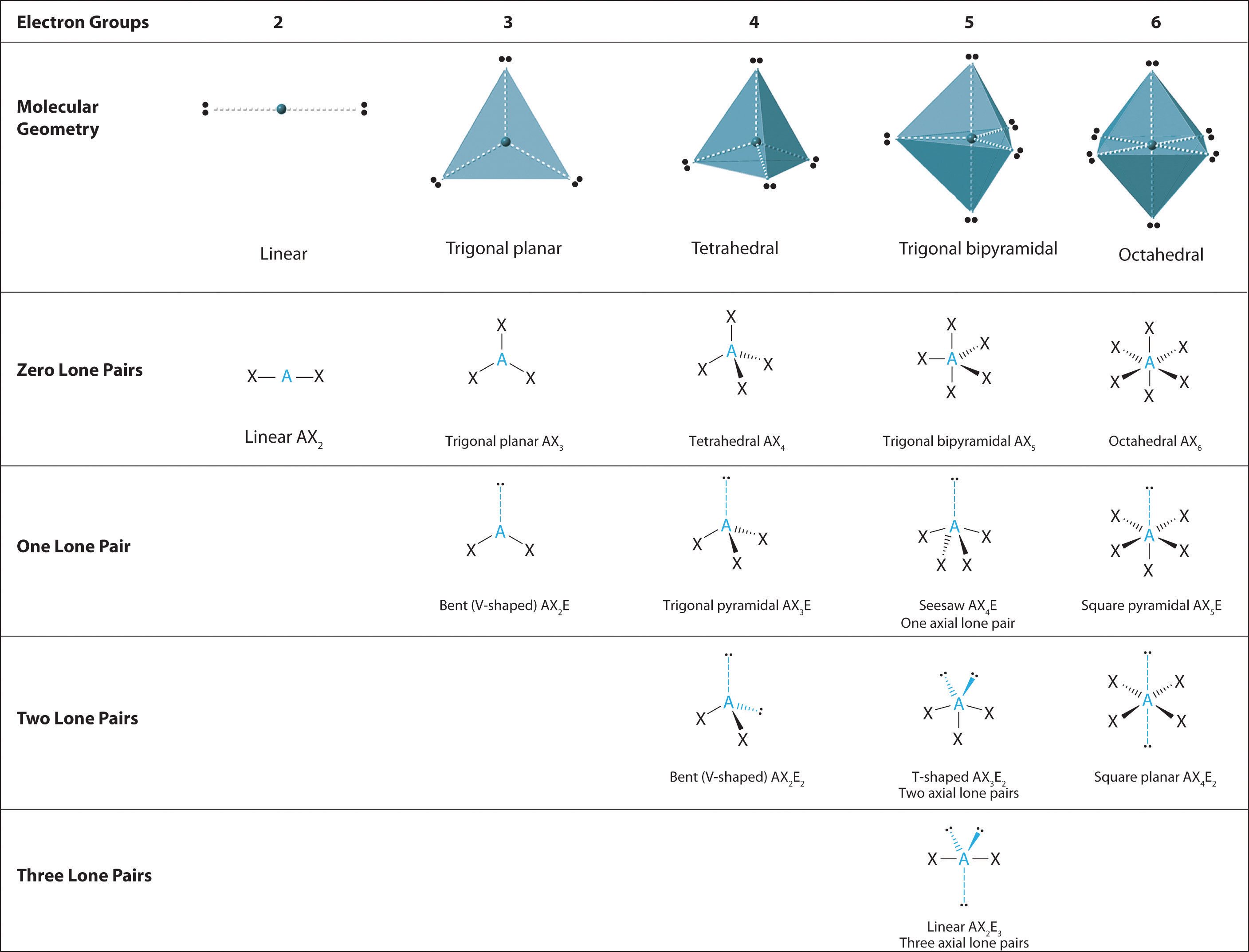
9.2: The VSEPR Model - Chemistry LibreTexts

VSEPR theory
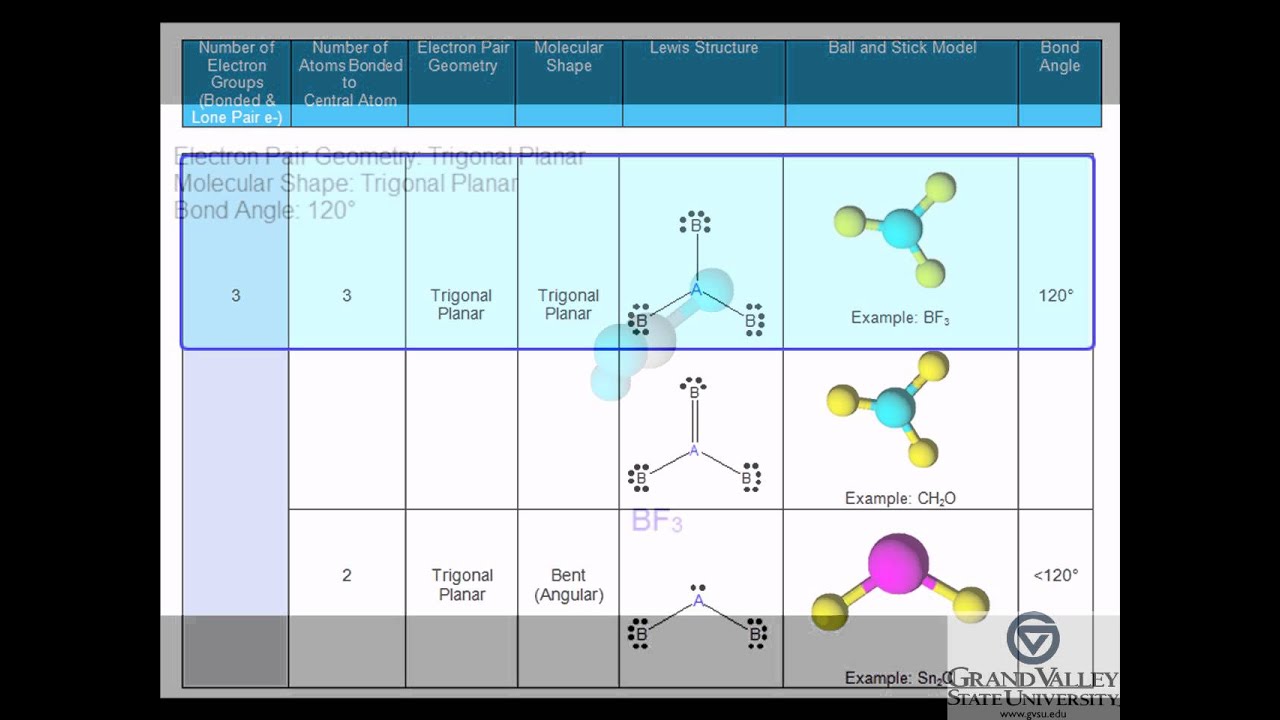
Molecular Geometry and Polarity: Trigonal Planar (nonpolar)

Valence-Shell Electron-Pair Repulsion Theory (VSEPR)

Molecular Geometry
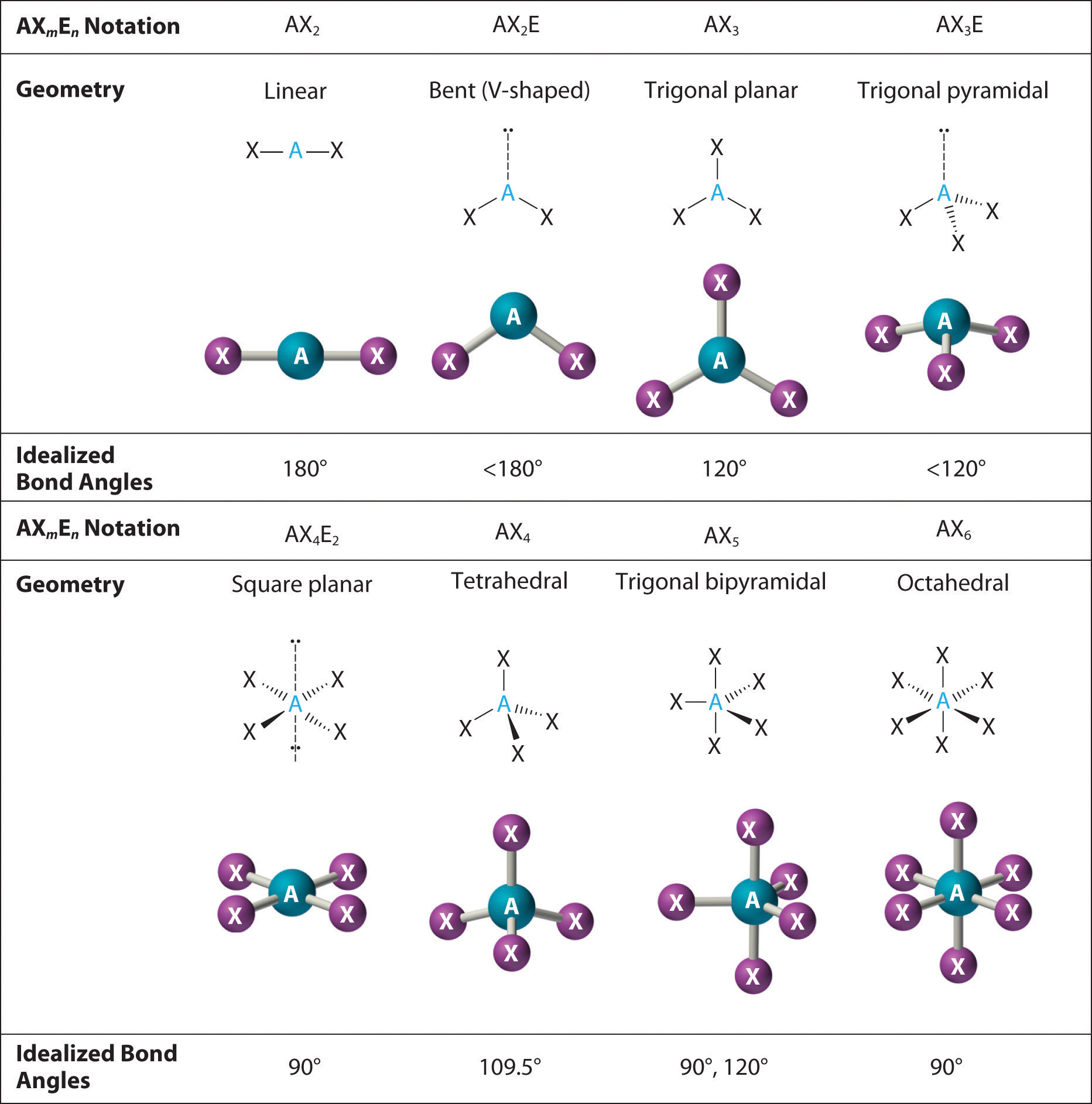
9.7: The Shapes of Molecules - Chemistry LibreTexts
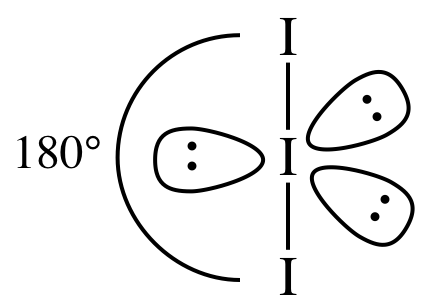
Section 11-1: Molecular Geometry: Using VSEPR Theory to

Calaméo - Test Bank For Chemistry A Molecular Approach 2nd Edition
de
por adulto (o preço varia de acordo com o tamanho do grupo)







